Modulus of a Complex Number
You probable depend upon rechargeable batteries each day to energy such things as mobileular phones, computer computers, and different small digital devices. When the battery runs down, what do you do? Plug it returned in, right? Have you concept approximately what truly takes place while you plug it in? How does it recharge? Batteries offer electric strength with the aid of using using a chemical response, however finally it slows down due to the fact the reactants emerge as used up.
To recharge the battery, you want to opposite the response. This calls for an enter of electrical strength, that’s why you need to plug within side the battery to recharge it. While it is recharging, the battery acts as an electrolytic mobileular. In an electrolytic mobileular, electric strength is used to provoke an oxidation discount response that would not spontaneously occur. Electrolytic cells aren’t most effective used to recharge batteries, however additionally to split natural metals from metal compounds, to split different chemical compounds (like water), and to electroplate metals. Let’s examine little nearer at precisely how electrolytic cells paintings.
Electrolytic Cell Parts
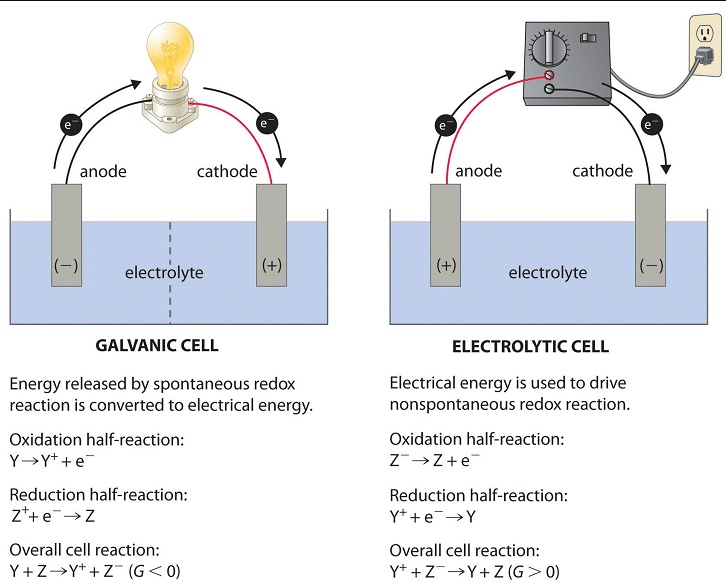
All electrolytic cells incorporate 3 primary parts: stable electrodes (referred to as the cathode and the anode) and a liquid electrolyte solution. The electrolyte solution conducts strength as it carries dissolve ions which can unfasten to transport round during the solution. The cathode and anode in an electrolytic mobileular connect to a supply of electrical strength, like a battery.
In an electrolytic mobileular, the cathode is usually negatively charge and the anode is positive charge. These electrodes are create from materials, consisting of copper, silver, and zinc, that take part within side the chemical response. These are refer to lively electrodes. They also can manufactured from chemically inert materials, like graphite, silicon, or platinum.
How Electrolytic Cells Work
So, now that we recognize the parts, let’s consider how all of them paintings together. What truly is going on in an electrolytic mobileular? First, the battery gives a supply of electrical strength, pushing electrons onto the cathode and making it negatively charged. Electrons also are draw out of the anode, making it undoubtedly charged. Once this takes place, an oxidation-discount response is activated.
At the anode, an oxidation response occurs, liberating electrons that then are draw to the undoubtedly charge anode. Meanwhile, on the cathode, a discount response occurs, which makes use of up the electrons which can be constructing up at the cathode.
Applications of Electrolytic Cells
The number one utility of electrolytic cells is for the manufacturing of oxygen fuel online and hydrogen fuel online from water. They also are use for the extraction of aluminum from bauxite. Another wonderful utility of electrolytic cells is in electroplating, that’s the manner of forming a skinny shielding layer of a selected metallic at the floor of any other metallic. The electrorefining of many non-ferrous metals is carries out with the assist of electrolytic cell.
Read Also: Simple diffusion- definition, precept, examples, applications
Such electrochemical cells also are use in electro winning processes. It may be refer to that the commercial manufacturing of high-purity copper. Also high-purity zinc, and high-purity aluminum is nearly usually carries out via electrolytic cells. To examine extra approximately electrolytic cells and different essential principles in electrochemistry. Sign in with BYJU’S and down load the cellular utility to your smartphone.


