Of course, students often worry about their chemistry marks going low in school. In order to learn chemistry in its totality, one needs to keep their ideas clear. So, starting at the base level, it is primary to focus on the part about elements and how different atoms and molecules merge to form one.
/what-are-the-seven-diatomic-elements-606623-v3-5b562dab46e0fb0037fee8c7.png)
Here, we can say that elements can either be monoatomic, diatomic, triatomic or, tetratomic.
In this article, we shall focus on the diatomic elements alone.
Diatomic Elements
Two atoms link to form a diatomic molecule. Monatomic elements, on the other hand, are made up of only one atom (e.g., Ar). However, we come across many diatomic elements in our daily life. For example, chemicals, such as HCl, NaCl, and KBr, are diatomic. Two distinct elements make diatomic compounds . 7 pure elements make diatomic molecules.
So, we can explain it more clearly.
In fact, diatomic elements are things that merge to form molecules made up of two atoms. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine are the seven diatomic elements.
In many ways, these elements can dwell in their purest form. For instance, oxygen stays as ozone, a triatomic molecule.
As halogens are a unique form of nonmetallic element, all of these elements are nonmetallic. At room temp, Br is a liquid, and the other elements are all gasses under normal state. The other elements form diatomic liquids as the temp. or pressure is reduced.
However, it is not true that these seven items alone can be diatomic.
Other ones can make diatomic molecules, but just these seven elements do so on a daily basis. Other elements’ diatomic molecules, on the other hand, are not really stable, and their bonds are designed to break.
Since the atoms form in pairs, we name such elements diatomic elements. H2, N2, O2, F2, Cl2, Br2, and I2 are the chemical formulae for these elements.
The diatomic elements are molecules that seem to have two atoms. A chemical link occurs between two atoms.
Diatomic elements definition
If you look all around yourself, you will note the earth comprises three basic things: nitrogen, oxygen, and a hint of argon. Now, to be more explicit, N2 accounts for 78% of the atmosphere, while O2 accounts for 21%. Both oxygen (O2) and nitrogen (N2) are diatomic elements, which implies they are made up of 2 atoms.
Diatomic elements are seven distinct forms made up of two atoms that form as pure elements.
This main group is made up of gasses, as a whole. When we look at the prefix ‘di-‘ in the word diatomic, we can get it from the Greek word ‘two.’ Check the formula to see if you’re dealing with a diatomic element. Every molecule has its own molecular formula, and diatomic elements’ formulas always have a subscript of 2, showing that its form has two main atoms. The diatomic element oxygen, for example, has the formula O2, showing that there are two distinct oxygen atoms active.
Read Also: What is the centripetal acceleration formula ?
Diatomic elements are unusual in that the atoms that do not exist alone. For example, you can never find a N or F atom alone. Yet, because they need to merge forces to get enough electrons, these atoms will always be linked together. Also, you can use this set mnemonic scheme to learn which atoms make up the 7 diatomic elements: “I Bring Cookies For Our New Home”.

If you need to find these diatomic elements just on the periodic table at any time, remember the ‘seven rule’ for sure. And, you can get the info easily.
Diatomic Elements meaning
The noble gasses ( For example, He, Ne, Ar, Kr, Xe, and Ra) are also monatomic gasses at Standard temp and pressure conditions. To clear them from many other gasses that are chemical forms, people call the homonuclear diatomic gasses and noble gasses as “elemental gasses” or “molecular gasses.”
For instance, the halogens bromine (Br2) and iodine (I2) also make diatomic gasses at little high temperatures.
Also,except for astatine and tennessine, all halogens have indeed been shown as diatomic molecules.
When you evaporate other elements, they produce diatomic molecules. Then, these vapors polymerize again when you cool them. P2 is made by heating (or “cracking”) pure P. The bulk of sulfur vapor is disulfur (S2). In the gas phase, dilithium (Li2) and disodium (Na2) are known. Also, in the gas phase, ditungsten (W2) and Mo2 create sextuple bonds. Moreover, Rb2 is a diatomic element.
Of course, from time to time people have seen many diatomic molecules in the Earth’s space, labs, and cosmic space. The Earth’s atmosphere is based on two types of diatomic molecules: First, N2 (78 percent) and then, O2 (29 percent). Now, hydrogen (H2) is the most common diatomic molecule in the universe. Thus, its natural plenty in the Earth’s space is in the range of parts per million. H2 atoms rule the galactic space.
The bond length or the direct space between the two atoms is the only factor that can separate all diatomic molecules. For example, the diatomic N2 atom has a triple bond, the diatomic O2 atom has a double bond. And, the diatomic H2, F2, Cl2, I2, and Br2 atoms all have single bonds.
Diatomic elements in periodic table
As some of the most basic elements, also H2, O2, and also N2, form as diatomic molecules. Of course, diatomic elements played a key role in the 19th century show of the ideas of element, atom, and also the molecule. The first atomic theory made by John Dalton held that all elements were monatomic. And, that atoms in compounds had the basic atomic ratios with regards to each other. For example, Dalton felt that water had the formula H2O . So, it would mean that the atomic weight of O2 was eight times that of H2. But, the real value is more or less 16. As a result, there was a half-century of dispute over atomic masses and molecular formulae.
Gay-Lussac and von Humboldt proved in 1805 that water is made up of two volumes of H2 and one volume of O2. And, Amedeo Avogadro came at the right view of water’s form in 1811, based on what is now known as Avogadro’s law and the theme of diatomic element molecules. Yet, people mostly did not pay heed to these results until 1860. In fact, this was partly because people thought that atoms of one element would have no chemical kinship for atoms of the same element. And, also partly because of clear threats to Avogadro’s law. But, that was not made clear until later in ideas of detaching molecules.
H, N, O, F, Cl, and Br, and I are the seven diatomic elements (I).
Since the atoms stay in pairs, we name them diatomic elements. And, these elements’ chemical formulae are H2, N2, O2, F2, Cl2, Br2, and I2.
Diatomic elements examples
Now, we have once given the seven main diatomic forms in the last part of the article.
So, we are going to list these again. For example,
- Hydrogen (H2)
- Nitrogen (N2)
- Oxygen (O2)
- Fluorine (F2)
- Iodine (I2)
- Chlorine (Cl2)
- Bromine (Br2).
They harden into diatomic forms when people cool them to a low temp. ALso, the hcp structure is found in N2, whereas you can find the hcp structure in O2.
However, the crystal forms of substances that are not metals, covalent, or diatomic are the most striking. Although boron (B) and sulfur (S) have various crystal structures, each has a basic style. Twelve boron atoms merge to form an icosahedron-shaped form Here, one can pile the molecules to form crystals.
Diatomic elements acronym
Just like we said before, hydrogen, oxygen, nitrogen, chlorine, bromine, iodine, and fluorine are the diatomic elements. You can pronounce the acronym HONClBrIF as honk-le-brif. Moreover, it has the atomic symbol for every one of the diatomic elements. So, it iis one way to recall the diatomic elements.
Diatomic elements are clear elements that come together to form molecules made up of two atoms fused as one. H, N, O, F, Cl, I, and Br are the seven diatomic forms. In many cases, these elements can live in their pure form.
The diatomic elements have all of the elements in the acronym HOFBrINCl (H, N, O, Br, F, I , and Cl). When people state these elements in a chemical form, they must bear the subscript 2. In fact, it is because people think that they are always diatomic.
Mnemonic: Have No Fear Of Ice Cold Beer.
Have No Fear Of Ice Cold Beer. H, N, O, I, F, Cl, I, and Br are our seven diatomic elements. What we like best about this mnemonic is that you will see that ice is a solid and beer is a liquid.
Diatomic elements Formulas
We are always seeing the periodic table of elements. 7 of those elements occur as diatomic molecules. So, the formula is not only the symbol, but also the symbol-subscript 2. They are like: At 25 °C– name of element– formula,
- hydrogen H2 gas
- nitrogen N2 gas
- oxygen O2 gas
- fluorine F2 gas
- chlorine Cl2 gas
- bromine Br2 liquid
- iodine I2 solid
Diatomic elements in chemical equations
On the periodic table, people can find the diatomic elements very easily. They consist of the halogens (F, Cl, Br, I) as well as O and N. These forms are near to one another on the periodic table image. H is unique from either diatomic element in the periodic table.
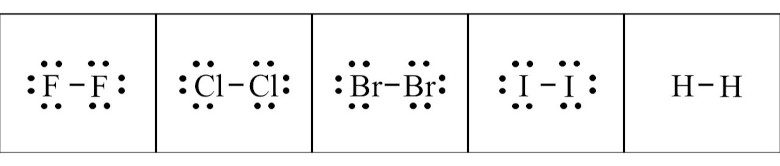
The diatomic elements are molecules in the form of elements. A chemical tie is between the two atoms. You can show F2, Cl2, Br2, I2, and H2 dot structures with ease. The horizontal line shows a link between the two atoms. And, you can show it using letters. Because of the octet rule, atoms are more robust in pairs.
The form of the diatomic elements oxygen and nitrogen is even more complex. In the O2 dot form, it is a double bond between the two O atoms. On the other hand, in the N2 dot form, there is a triple bond between the two N atoms.
Aside from Noble Gasses, all of the basic gasses are bimolecular, that means they are diatomic molecules. Consider that N2, O2, F2, and Cl2 are all diatomic gasses.
Except for Br(A liquid), and I, which is a gas, all halogens live as diatomic molecules. Inorganic scientists will often refer to biomolecule gasses as dinitrogen and dihydrogen to clearly show their molecularity.
There are also a number of heteronuclear diatomic compounds, such as H X, N O, and CO.
Diatomic elements class 9
Of course, career experts have kept diatomic elements in the scope of the syllabus of class 9. In fact, this is the basic build for chemistry in higher classes. We can define diatomic molecules as forms made by the mixing of two atoms which might be either same or unlike chemical elements.
We define diatomic molecules as molecules formed of two atoms of the same or other chemical components. The prefix di- denotes “two” in the term diatomic shows it perfectly. When you get a diatomic molecule from the same chemical element, it is called homonuclear. And, they are heteronuclear when two different chemical elements form the diatomic molecules.
As noble gas elements ensure a steady electronic config., they are monatomic. Thus, they do not form a diatomic molecule.
Diatomic molecules have a linear geometry. And, you can distinguish them by the space between the two atoms.
Two hydrogen atoms merge to form the H2 molecule. As a result, H2 is a diatomic molecule. As both components in the molecule are the same, it is a homonuclear molecule.
Two Cl atoms make up the Cl2 molecule. As a result, the Cl2 molecule is a diatomic molecule. Because both components in the molecule are the same, it is a homonuclear molecule.
Two fluorine atoms make up the F2 molecule. As a result, the fluorine molecule is a diatomic molecule. Because both components in the molecule are the same, it is a homonuclear molecule.
Diatomic elements FAQs
1. How many diatomic elements are there in the world?
Ans: Diatomic elements are mainly stable forms that react to form molecules. Two same atoms fuse together to form such molecules. H, N, O, F, Cl, I, and Br are the seven diatomic elements. In various cases, these elements can exist in their purest form.
2. Why do diatomic molecules exist?
Ans: We can make a diatomic element using two same atoms. You can get the term diatomic from the words ‘di’ (two) and ‘atomic’ (atom). With only one atom, a monatomic element is stable. As it helps them to follow the octet rule, these diatomic elements are most robust in this paired form.
3. Are diatomic elements ever alone?
Ans: You can form carbon monoxide gas CO when an O bonds with a C. In fact, Oxygen atoms (O) must ever link to something else. So, they can not be alone with each other. The same is true for all seven diatomic elements.
4. Can we break down diatomic elements?
Ans: While only the seven elements form diatomic molecules on a regular basis, other elements can do that as well. Other elements’ diatomic molecules, on the other hand, are not very durable, and you can break their bonds easily..
5. What is the smallest particle of a diatomic element?
Ans: Of course, the smallest particle of a diatomic element is the same as any other element in general.
So, an atom is the tiniest part of an element, with about the same chemical properties as the main element.
6. Why are diatomic molecules gasses at room temperature?
Ans: At temp. low enough to turn them into liquids, O2, N2, and other diatomic molecules that really are gasses at normal temperature remain diatomic. When low temp. slows the particles down a lot, factors less than atomic bonds that bind surrounding molecules let them reach the liquid state.
7. Are diatomic elements polar or nonpolar?
Ans: Any diatomic molecule with the same element in both atoms has to be a non – polar molecule. But, a polar molecule is a diatomic molecule with a polar covalent bond, such as HF.
8. Can we consider water to be a diatomic molecule?
Ans: Water is not a compound because only one element atom – oxygen forms it. We call this form of molecule a diatomic molecule, which is made up of two atoms of the same type
9. Can diatomic molecules be ionic?
Ans: Ionic chemicals do not form diatomic molecules under standard conditions. In fact, most exist at ambient temperature as many tiers of ions are put in crystal lattices.